
A Beginner's Guide to pH Management
- Target pH: 5.5 - 6.5 (ideally 6)
Your pH tells you how acidic or alkaline your nutrient is.
Get it wrong, and some minerals in your solution will be unavailable to your plant.
Let us explain.
What's pH?
Your pH tells you how acidic or alkaline your solution is on a scale of 0 - 14
Really, that's all you need to know, but if you feel like nerding out, here's a bit more info.
- pH = potential Hydrogen
Your pH really tells you the Hydrogen (H+) to Hydroxide (OH-) ratio
- H+ causes acidity
- OH- causes alkalinity
Think of water...
Water is H2O
It has one of each ion, so is pH neutral.
Water that comes out of your tap won't quite be pH neutral, because minerals have been added to it.
At a glance, it doesn't look like there's that much difference between each pH.
Don't be fooled. The difference is huge (pH 2 is 10,000 x more acidic than pH 6)
| pH | H+ to OH- ratio | ||
| Alkaline | 14 | Drain cleaner | 1/10,000,000 |
| 13 | Bleach | 1/1,000,000 | |
| 12 | Quicklime | 1/100,000 | |
| 11 | Ammonia | 1/10,000 | |
| 10 | Milk of magnesia | 1/1,000 | |
| 9 | Baking powder | 1/100 | |
| 8 | Eggs | 1/10 | |
| Neutral | 7 | Pure water | 1/1 |
| 6 | Milk | 10 | |
| 5 | Bananas | 100 | |
| 4 | Tomato juice | 1,000 | |
| 3 | Orange juice | 10,000 | |
| 2 | Lemon juice | 100,000 | |
| 1 | Stomach acid | 1,000,000 | |
| Acidic | 0 | Battery acid | 10,000,000 |
Why does it matter?
As a rule, plants best absorb nutrients when your feed is between pH 5.5 - 6.5.
If you drift outside this range, some nutrients can precipitate out of your solution. This is where nutrients in your solution become solids.
When this happens, they can no longer be absorbed by your plants (more on precipitation here).
- A low pH can cause Magnesium lockout
- A high pH can cause a Nitrogen deficiency
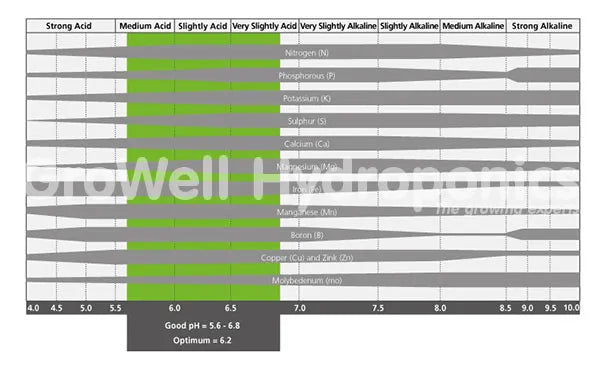 All plants have slightly different pH preferences, but for most, pH 5.5 - 6.5 is good.
All plants have slightly different pH preferences, but for most, pH 5.5 - 6.5 is good.
| Recommended pH | |
| Strawberry | 5.8 - 6.0 |
| Lettuce | 5.6 - 6.0 |
| Tomato | 5.5 - 6.4 |
Setting your pH
Most people just alter their pH once, when they first mix their nutrient.
The only time you don't need to do this is in soil - it naturally buffers your pH.
1. Dechlorinate
Chlorine affects the pH of water, so you need to dechlorinate.
Leave your water to stand for 24 hours. Or better yet, use an RO Filter.
2. Add nutrients
Next, add all of your nutrients and boosters.
Add each one separately and mix well in between.
Be sure to add SHOGUN Silicon - it buffers your pH and strengthens plants.
3. Test
Measure your pH.
There are lots of tools for the job - the most popular are:
4. Tweak
Add pH Up or Down to tweak. The trick is to not overdo it.
Dilute a little pH with water, then add a drop at a time.
If you add too much, correct with water.
Maintaining your pH
As plants use minerals and nutrients, your pH can drift up.
It's tempting to tweak it every day. Don't. pH solutions are aggressive and best used sparingly. Overdo it and you can get nutrient lockout.
At most, add pH every other day.
If you find that you're having to use too much pH Down, try switching feeds.
- Soft water feeds: Don't affect your pH
- Universal feeds: Bring your pH down slightly
- Hard water feeds: Really reduce your pH
| Water type | ||
|---|---|---|
| Hard | Soft | |
| pH | 7.8 or higher | 7.7 or lower |
| CF | 8 or higher | 7 or lower |
Safety & Storage
Right, here comes the safety spiel...you knew it was coming.
1) Keep all aggressive chemicals under lock and key
2) Keep out of the reach of children
3) Wear goggles, gloves and appropriate safety clothing when handling aggressive liquids
4) Never mix pH Up and Down
5) Store pH Up and Down separately
| Hint |
| To prolong the life of your pH equipment, keep the probes hydrated with KCL solution. You just add it to the cap of any probes and pens during storage and job's a good'un. |









